Gene editing produces breakthrough canola variety
Shorter, highly branched canola plants with more pods show the power of the cutting-edge technology
| 4 min read

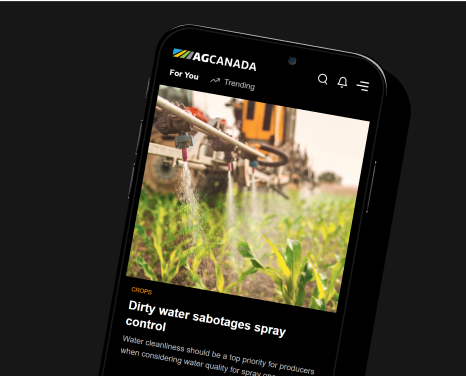
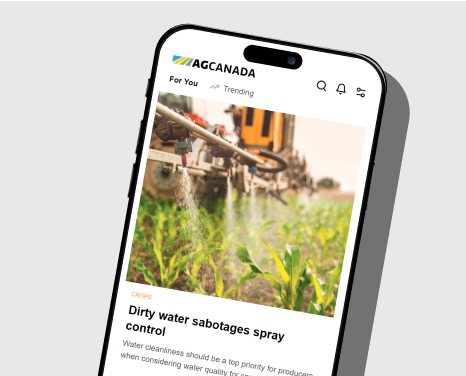
Having proven gene editing can produce shorter canola that would be subject to less lodging, U of Calgary researchers are now using this cutting-edge technology to reduce pod shatter and boost protein content in canola, says Professor Marcus Samuel. Photo: Supplied
University of Calgary researchers have used gene editing to bring to life a new shorter, highly branched variety of canola that has more pods and is easier to harvest.
“Based on my conversations with some people in the agriculture industry — including primary producers — they would love to have a crop like this,” said U of Calgary cell biology professor, Marcus Samuel. “There’s definitely a need for something like this.”
The new cultivar’s short stature (it’s 34 per cent shorter than most canola) is intended to minimize lodging — a major selling point in itself — and the extra branches mean more flowers and pods.
“The problem with canola is that we have no control over how tall it grows,” said Samuel. “It’s usually more than a metre (high) and that makes it very prone to lodging.”
Inspired by the Green Revolution of the 1960s — which saw the breeding of shorter, more compact and nutrient-efficient varieties of rice and wheat — Samuel, grad student Matija Stanic and other researchers used CRISPR/Cas9 technology to develop this line.
They targeted a hormone called strigolactone, which is responsible for shutting down branching in the plant. They used CRISPR/Cas9 — often described as a genetic ‘scissors’ that can cut unwanted traits from the DNA of a plant — to knock out receptors that perceive strigolactone.
The gene-edited canola does not yet have a name and was never intended to be commercially developed.
Rather, it is a proof of concept and Samuel said some seed companies are interested. Such a partnership would require access to the company’s germplasm.
“We need something that the industry will actually use,” he said. “If we can get their germplasm we can cross this trait into that germplasm so we can test it out to see if there’s an increase in yield.”
The process has taken 3-1/2 years but there is more work to be done. First and foremost is determining if there are unintended side-effects.
“There could be a lot of other things that are shut down in the absence of perception of this hormone,” said Samuel. “We’re looking at where we can target branching and plant height exclusively without compromising any other things.
“Sometimes root growth might be compromised when you shut down things like that. So we’re trying to find other candidates that we can go after to duplicate what we’ve done which may be better than our current line.”
One “drawback” has been identified.
“Under extreme drought conditions where there’s complete lack of water, we see these lines being a little bit more sensitive than the unmodified control lines,” he said.
“So if we go after the other downstream candidate gene in the pathway we could exclusively target branching and the height of the plant without touching any of the other phenotypes (observable traits).”
Nevertheless, it’s groundbreaking work and could not have been produced by way of conventional breeding — at least not without luck, said Samuel.
“It’s like a microsurgery,” he said. “We used the enzyme and went into the canola cells and then made these minor snips in the genes you want to knock out.
“In this case we were knocking out the gene that is important for producing the protein that perceives this hormone. This hormone is important for shutting down branching.
“We were able to knock down all four copies of the gene in canola and that led to the highly branched plant we had anticipated.”
Traditional breeding would have required starting with a short, highly branched variety of canola “but you don’t find that in nature” unless you happen across a mutation, he said.
U of Calgary researchers have already turned their sights on other canola traits that could be improved with gene editing.
“We have a number of projects looking at shatter tolerance in canola, which is a bigger problem,” said Samuel. “We also have projects to improve seed protein content in canola. Both projects are ongoing in the lab currently.”
And although commercial varieties of canola created by gene editing are still years away, Samuel’s breakthrough comes only months after Health Canada — after several years of deliberation — declared gene-edited crops safe for human consumption and the environment. This is an important step for the future of gene editing in the country, said Samuel.
However, gene-edited food products still face an upward battle in terms of acceptance. They are still considered GMOs in many countries (notably China and the EU countries) even though they don’t contain any transgenic material.
“When it comes to exporting, that means we need to know what the policies of those countries are before we actually start the work,” he said.
However, Samuel is optimistic this technology will eventually gain mass acceptance.
“I can confidently say that within a couple of years you will see most people accepting it because this microsurgery we’re talking about leaves no trace of any foreign gene in there when you’re finished working with it, so it should be completely safe,” he said. “It’s very similar to a natural selection process where you get a mutant plant.”
This album was originally published at the Alberta Farmer Express.